Development of a Biodegradable, Biocompatible Tympanostomy Tube Using an In-Vitro Ear Chamber
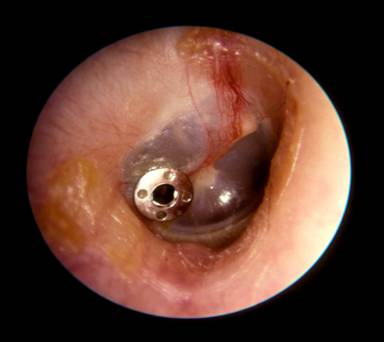
Ear infections are the leading cause of hearing loss in children, and the number one reason for children to be given an anesthetic. Ear infections are formed from mucus that builds up within the middle ear space, and causes pain and hearing loss. To relieve these symptoms, tympanostomy tubes (Figure 1) are inserted into the ear drum to remove the mucus. Approximately 2 million children receive tympanostomy tubes each year, and many require several sets of replacement tubes.
Tympanostomy tubes become plugged in ~30% of cases and new developments in tube coatings, and compositions have been created to prevent these blockages. An in-vitro ear chamber can be used to determine the effectiveness of each design without using patient studies. This chamber reduces the number of testing variables, is inexpensive, and can test tubes in a matter of hours as opposed to months for patient studies.
Furthermore, the need for the development of a biocompatible, biodegradable tympanostomy tube has increased over the past 10 years. Current tympanostomy tubes are afflicted with many drawbacks including tube plugging, and residual ear drum perforation. The use of more biocompatible substrates in the formation of tympanostomy tubes can reduce the prevalence of tube plugging and ear drum perforation. This can be assessed by placing newly designed tympanostomy tubes into an in-vitro ear chamber and measuring the formation of plugs.

Fig.1: Metal tympanostomy tube inserted in the ear drum.