Impact of Shear Stress on Early Vein Graft Remodeling: A Biomechanical Analysis
|
| |
|
| Arterial occlusive
disease, causing myocardial infarctions and strokes, affects millions of
people and is one of the leading causes of death in the United States.
In many cases, invasive surgical techniques, such as bypass vein
grafting and angioplasties, have been used to alleviate vascular
occlusions. Bypass vein
grafting uses a vein taken from another part of the body to replace the
obstructed blood vessel and inserts it into the arterial system to provide
better blood flow. However, restenosis or occlusion can still occur
in the time frame of months to years. Because of these reasons, many
researchers have been attempting to improve these long-term patency
results. An understanding of
early vein graft adaptation and progression must be established in order
to improve the long-term results. Currently,
it is known that many factors, including physical forces, morphologic
changes, and biochemical events, are involved in this adaptation process.
Playing a key role in the remodeling process are the biomechanical
forces. The changes in these
biomechanical forces regulate the balance between intimal thickening and
expansive remodeling, which govern the morphologic changes in the vein
graft. The objective of this study is
to characterize early vein graft remodeling by finding the correlation
between the physical forces and morphologic changes by characterizing the
dynamic shear and wall tensile forces with the intimal thickening and
expansive remodeling that occurs in vein graft arterialization. |
| Previously ex vivo
models were used to determine the hemodynamic forces in the steady state
only. Our mathematical model
uses an in vivo bilateral carotid vein graft with distal branch
ligation model to collect the experimental flow rate data in a pulsatile
hemodynamic environment. The
experimental animal model created two flow environments with reduced
flow/shear through the ligated vein graft and elevated flow/ shear in the
contralateral vein graft (Figure 1). |
|
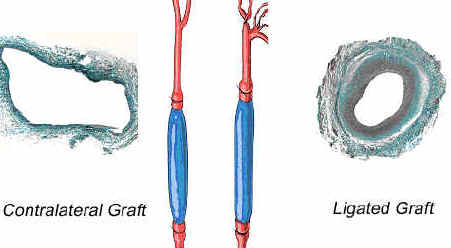
Figure 1. The effects of low and high wall tension
on a vein graft. |
|
| Using New Zealand white male rabbits, vein
grafts were implanted and then harvested at 1, 3, 7, 14, and 28 days after
initial surgical procedure. Hemodynamic
and video measurements were collected before and after ligation for
calculation of the shear and wall tensile forces.
A computational program was developed to determine the velocity and
shear stress profiles based on the collected hemodynamic data. Due to the size and length of the rabbit vein graft model
(Figure 2),
the pressure gradient is difficult to ascertain; therefore the flow
measurement is used for the basis of our calculations.
This approach is slightly different from previous models that
relied upon the derived pressure gradient. |
|
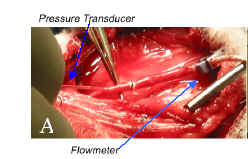
Figure 2. (A) Measurement of in vivo flow
rate and pressure in rabbit vein graft. |
| Vein grafts were exposed to distinct flow environments
characterized with a 6-fold difference in mean flow rate.
Accelerated intimal hyperplasia and reduced outward remodeling were
observed in the low flow grafts. At
day 7, there was a peak in maximum and minimum shear stress with a delayed
increase in lumen diameter leading to normalization of wall shear by day
28. At day 3, the intramural
wall tension was at its maximum and there was an increase in wall
thickness leading to a significant reduction of these stresses by day 14.
There was no difference in incremental modulus of elasticity
despite the significant difference in remodeling between the high and low
flow grafts. |
| Our mathematical model provides a simple way to determine
dynamic wall shear and tension in a pulsatile hemodynamic environment,
using readily available technology. Our
mathematical model reveals a correlation among shear stress, flow, and
intimal thickening, which coincides with ex vivo studies modeling
steady flow. Current research
is working towards a more realistic development of computational modeling of
pulsatile blood flow in this model as well as other more complicated
configurations such as bifurcated blood vessels, which in the past have
dealt with steady flow in ex vivo models. |
|
| |
| 3-D movies of the velocity and wall shear stress in a vein graft under a pulsatile high flow environment: |
| The following movies illustrate the velocity and wall shear
stress profiles in a rabbit vein graft under high flow conditions over one cardiac
cycle. The wall shear stress movie shows the wall shear stress profile vs. the diameter of the vein graft over one cardiac cycle. The velocity profile shows the velocity vs. the diameter of the vein graft over one cardiac cycle. |
| 3-D movie of shear stress profile
under high flow conditions |
| 3-D movie of velocity profile
under high flow conditions |
|
| For more information on the experimental rabbit vein graft
model:
Jiang Z., Wu L, Miller BL, Goldman DR, Fernandez CM, Abouhamze ZS,
Ozaki CK, Berceli SA. A novel vein graft model: adaptation to
differential flow environments. Am J Physiol. Circ. Physiol. 286:
H240-H245.
|
|

